The N-methyl-D-aspartate receptor (NMDAR) non-competitive antagonist (R,S)-ketamine has been used in human and veterinary medicine as a safer and short-acting alternative general anesthetic to phencyclidine (PCP) since the approval of intravenous and intramuscular ketamine hydrochloride in 1970 by the FDA. It is used in very high doses in this realm and causes a dissociative effect that allows painful procedures without recall or pain while preserving respiratory drive. It supports the cardiovascular system and facilitates general anesthesia in patients who cannot mentally handle IV induction. Because at lower doses it acts as a NMDA receptor agonist, it is very important in the treatment of chronic pain by effectively closing the channel for ion transport, disallowing transmission of the pain signal. This process gives the neuron a chance to recover and enable more normal transmission. This led to the development of drugs based on it as antidepressants and anti-anxiety drugs, which are now also approved by the FDA – intranasal form of the drug under the brand name SpravatoTM (Janssen Pharmaceuticals), a dissolving wafer placed under the tongue Wafermine TM (iX Biopharma). Nowadays ketamine is gaining ground as a promising treatment for pain syndrome, major depression, anxiety disorders, PTSD, OCD, addictions, including acute opioid withdrawal and other mood conditions. Ketamine can be given through several routes including intravenous push or infusions, intramuscular, intranasal, and orally. Investigations have mainly utilized IV infusions due to the precise dosing and ability to adjust if known side effects occur. In clinical practice, all these types of ketamine are used, since invasive methods are not always convenient.
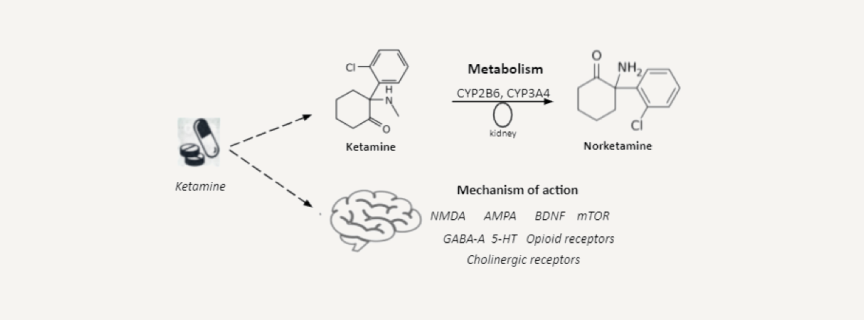
What is the mechanism of action?
According to its physicochemical properties, ketamine is a racemic mixture of the enantiomers of R-ketamine and S-ketamine, while the latter has a greater anesthetic effect than R-ketamine and is quickly eliminated. The data on the ratio of their dissociative characteristics are controversial; direct comparisons between S-ketamine and R-ketamine or racemic ketamine have not been carried out in a clinical setting, so it is impossible to make an informed choice in favor of one of the enantiomers or racemate for the treatment of depression. Unlike traditional medicines, targeting the monoamine brain pathways, ketamine has potent effects on the central nervous system glutamatergic system, in particular blockade of N-methyl-d-aspartate (NMDA) receptors, which play a significant role in depression, anxiety and trauma-related disorders. Ketamine can produce a hyperglutamatergic state, which persists long after elimination of ketamine from the blood and may contribute to increasing dendritic spine density, synaptogenesis and synaptic plasticity. Besides NMDARs and AMPARs (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors), ketamine interacts with a variety of other receptors and ion channels. Although these are typically lower affinity interactions, they nevertheless have potential to be involved in the antidepressant effects of ketamine. These include GABA-A, dopamine, serotonin/5-HT, opioid, and cholinergic receptors, in addition to voltage-gated sodium channels, L-typevvoltage-dependent calcium channels and hyperpolarization-activated cyclic nucleotide-gated channels.
What are the clinical effects of Ketamine?
Clinical data for different modes of administration indicate that 70% of responses and 30–40% of remissions are achievable for patients who have not responded to a range of modern treatments, including psychotherapy, and in some cases, ECT. It has been found that patients with severe, therapeutically persistent depression have approximately 70% positive response after a single infusion of ketamine. However, this effect is limited in time and after two weeks the symptomatology also recurs in about 70% of cases. The uniqueness of the main properties of ketamine is determined by its mechanism of action – the functional dissociation of stable connections between the thalamo-cortical and limbic structures of the brain. This circumstance explains the lack of a consistent dose-dependent effect of the drug. Dissociative effects preceding deep specific sedation are manifested in a rather narrow transition zone, approximately 1–1.5 mg / kg IM or 0.5–0.75mg / kg IV. Within these doses, the condition may be accompanied by sensory isolation when the patient does not respond to treatment and pain stimuli. In addition, the characteristics of psychedelic experiences and the ultimate antidepressant effect are influenced by the psychological readiness for positive feelings and the very conditions of the session. A valuable property of ketamine is its ability to have a beneficial effect on the suicidal trend of thinking and reduce suicidal activity in general. In addition, it was possible to show that anti-anhedonic effects are manifested regardless of the weakening of depressive symptoms, that is, ketamine has its own anti-anhedonic potential apart from the properties of an antidepressant. It is known that the antidepressant effects of ketamine, although they manifest themselves very quickly (often after the first procedure), with a single administration, persist for a short time, usually a day or two, less often for several days. The presence of alcoholism among family members of a depressed patient was found to be a positive predictor of the therapeutic effects of ketamine. The presence of an anxiety component of depression also suggests an additional benefit of ketamine prescription, as these patients often experience longer periods of symptom relief. This is not surprising since anxiety disorders themselves, such as PTSD and OCD, respond well to ketamine. Indications for prescribing ketamine can also extend to psychotic depression. Another example of the successful use of ketamine, in addition to its analgesic properties, in palliative care for patients with terminal cancer is the pacifying effect on the painful experiences associated with impending death.
Evidence of therapeutic efficacy
A double-blind, randomized, parallel-group, placebo-controlled trial has shown that a single intravenous infusion of ketamine dose-dependently improves depressive symptoms in patients with treatment-resistant depression. The onset of ketamine action reveals in a few hours after administration. Moreover, the effect of ketamine (0.5 mg/kg) is more pronounced with increasing depression severity. Phillips et al. performed single-site randomized double-blind crossover comparison aimed at evaluating of the antidepressant effects of a single ketamine infusion, a series of repeated ketamine infusions, and prolongation of response with maintenance infusions. They have shown that repeated ketamine infusions have cumulative and sustained antidepressant effects and once-weekly infusions can be applied to maintain the reduction of depressive symptoms. Corriger and Pickering have reviewed a huge amount of studies focused on the ketamine effect in depression including reports of intranasal (S)-ketamine administration. Briefly, in most studies ketamine provides a rapid antidepressant effect lasting for 1-2 weeks. This is especially interesting concerning patients experiencing suicidal thoughts because of the rapid effect in suicidal ideation elimination. Double-blind, randomized, placebo-controlled crossover trials in adults with DSM-5 social anxiety disorder (SAD) have shown that ketamine results in a significantly greater reduction in anxiety relative to placebo at 2, 5 and 10 days after a single injection. The number of treatment responders was significantly greater in the ketamine-administered group than in the placebo group. Glue and colleagues have also shown the anxiolytic effect of ketamine in double-blind psychoactive-controlled replication study in patients with treatment-resistant generalized anxiety (GAD) without depression. Ketamine dose-dependently improves anxiety ratings within an hour after administration and this effect persists for up to 1 week. A recent literature review by Banov et al. has summarized the treatment potential of ketamine in different types of anxiety disorders. Besides GAD and SAD, the review includes studies on obsessive-compulsive disorder, bipolar disorder with anxiety and post-traumatic stress disorder. Ketamine has been shown to improve anxiety-like symptoms in these conditions. To sum it up, ketamine appears to become a novel therapeutic strategy for the management of treatment-resistant patients with both depressive and anxiety disorders. It uniquely modulates glutamatergic brain system, synaptogenesis and synaptoplasticity, well tolerable, has minimal time lapse between infusion and onset of action, mild or moderate immediate side effects, which are transient.
Ketamine legal basis
On March 6th 2019, the United States Food and Drug Administration (FDA) approved a drug-device combination for intranasal (IN) esketamine under the brand name Spravato (Janssen Pharmaceuticals). IN esketamine is approved for a single indication: as an adjunct to oral antidepressants for treatment resistant depression in adults. Despite FDA-approval of the IN esketamine device, use of intravenous (IV) ketamine for depression remains off-label. Likely due to the absence of patentability, there are no plans to pursue FDA-approval of IV ketamine. However, the current expert consensus on the use of ketamine for depression acknowledges that this compound has enormous potential in patients with depression, even for those with mild to moderately resistant cases. The United States Drug Enforcement Agency (DEA) has categorized all forms of ketamine as schedule III, which is defined as having “moderate to low potential for physical and psychological dependence.” Prescribing of IN esketamine (Spravato) is further managed by its strict, limited distribution through its REMS program. The Spravato Risk Evaluation and Management Strategy (REMS) program requires individual prescribers, patients, and pharmacies to register with a centralized system before they are eligible to obtain and use esketamine. Also, Wafermine is developed under the US Food and Drug Administration (FDA). New Drug Application (NDA) pathway for acute moderate to severe pain. A phase 2b study in moderate to severe acute, postoperative pain confirmed that Wafermine has strong analgesic efficacy and is safe and well tolerated in patients. Although Wafermine is an unregistered drug, iX has been supplying Wafermine to hospitals in Australia since 2014 under a regulatory exemption for use by specialists to treat pain, with excellent results and safety. In addition to pain, Wafermine is ready for phase 2 trials in MDD. While oral antidepressants take weeks to start having an effect, the ketamine delivered by Wafermine takes only hours. As well as effectively treating TRD, Wafermine could be used as a bridging initiation therapy in more severe MDD.
Is ketamine safe?
The most common side effects of intravenous ketamine administration are transient tachycardia and arterial hypertension, less often nausea and vomiting.These side effects are usually well tolerated and rarely lead to discontinuation of treatment. Over the long history of the use of ketamine in anesthesiology, more than 10 thousand studies have been published confirming its safety in a wide range of doses in patients of different ages. Even in surgical practice, where doses are 10 times those used to treat depression, ketamine remains a safe drug. A review of over 70,000 reports on ketamine anesthesia has reported only one death in a patient with serious physical problems. Despite the fact that ketamine, due to its psychoactive effects, is potentially capable of provoking abuse and addiction, such cases are reflected only in a few cases of self-medication. Intravenous administration is the most commonly used, but its safety and efficacy have been established for other modes of administration, including oral, sublingual, transmucosal, intranasal, intramuscular, and subcutaneous. The use of subanesthetic doses of ketamine to overcome drug-resistant depression is a safe and effective therapy.
What are the contraindications to ketamine treatment?
Conditions that may limit your treatment include:
- If you have a history of substance abuse – such as alcohol or drugs – it’s especially important for you and your doctor to consider whether ketamine is a good option for you.
- Severe Personality Disorders (with caution)
- Delusional Disorder
- Bipolar disorder (with caution)
- Untreated HTN
- Cardiovascular Disease
- Kidney disease or impairment
At Hive, our ketamine therapy programmes will be individually selected for each patient. Ketamine therapy should only be administered under the supervision of a physician; not only will this increase the likelihood of achieving the desired long-term treatment results, but it will also minimize the risk of side effects.
The most common side effects during a ketamine experience are:
- A sense of impaired balance and coordination
- Drowsiness and mental confusion
- Visual, auditory, or tactile distortion
- A feeling of anxiety, fright
- Nausea, vomiting
